Colorful world of hydrogen fuel and its race to clean energy Beyond grey
The hope of all humanity for clean air is hydrogen - the most common chemical element in the Universe. It is contained in any matter in which it makes up about 75%. It is lightweight, non-explosive and can be stored anywhere and does not itself pollute the environment. All this makes it an almost ideal fuel source.
London's double-decker buses have been running on hydrogen for several years. About 10 years ago, Toyota released the Mirai sedan, powered by hydrogen fuel cells. However, extracting hydrogen and turning it into fuel requires a chemical and technological process. There are many ways to convert hydrogen into fuel, but they are unequal and have advantages and disadvantages.

This is how the colour classification of hydrogen appeared. Hydrogen itself is, of course, a colourless gas, but in the classification below, each colour corresponds to a different extraction process.
The most common types of hydrogen based on their production method are grey, blue and green hydrogen.
Grey
Gray hydrogen is currently the most common and cheapest method of hydrogen extraction. Gray hydrogen in this method is produced from natural gas using steam reforming, which separates the hydrogen from the natural gas. However, the technologies used do not capture the carbon emissions generated by the process and are instead simply released into the atmosphere.
Blue
Blue hydrogen is also recovered through the steam reforming process, but it differs from gray hydrogen in that the carbon emissions released are captured and stored, reducing emissions but not eliminating them to zero. Blue hydrogen is sometimes called "low-carbon hydrogen" because the production process does not eliminate greenhouse gases, but simply retains them.
Green
Green hydrogen produces no emissions throughout its entire production cycle because it uses renewable energy during the extraction process, making it a true source of clean energy. It is produced by electrolysis of water using environmentally friendly electricity generated from renewable wind and solar energy. This process causes a reaction that breaks water into its components: hydrogen and oxygen (H and O in H2O).
This results in no carbon emissions being emitted during the process. This is an excellent alternative to gray and blue hydrogen, but at the moment the main problem is the high cost of this technology. Today, scientists are trying to make it a truly accessible, environmentally friendly alternative to other methods of producing hydrogen. In addition to the three main types of hydrogen, there are a number of other colours, which are discussed below.
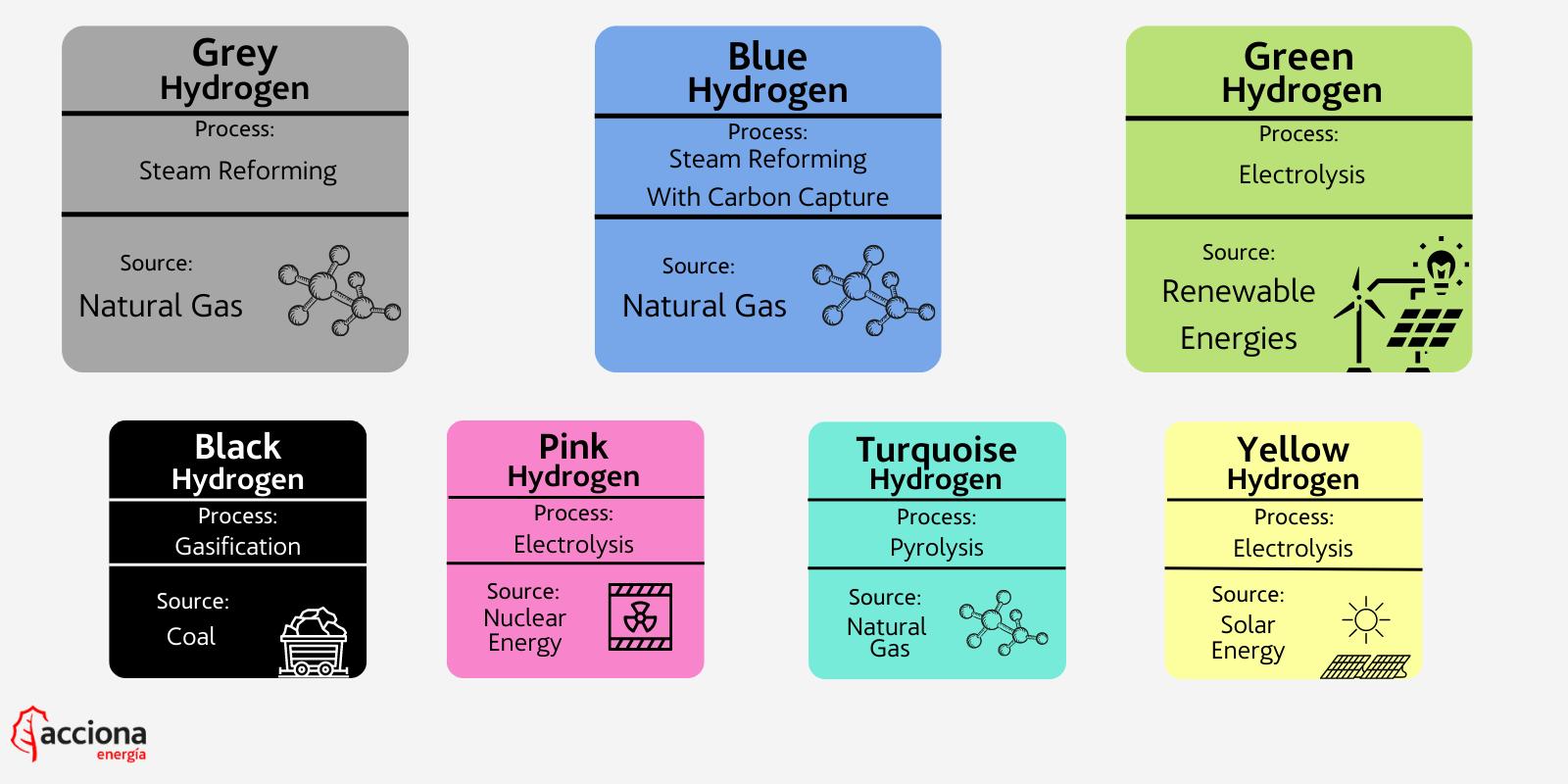
Black and Brown
Black and brown hydrogen is produced by processing using any type of coal. This process, called gasification, is the opposite process of green hydrogen electrolysis. This is a fairly traditional process used in many industries in which carbon-rich materials are converted into hydrogen and carbon dioxide. The emissions are then released into the air, making the hydrogen produced the dirtiest of all types.
Pink
Pink hydrogen is produced through electrolysis using nuclear energy. You may see other names for pink hydrogen, also called purple or red hydrogen.
Turquoise
Turquoise hydrogen is very new and is still being studied to see if it can be used effectively on a large scale. It is produced using a process called methane pyrolysis, which produces hydrogen and solid carbon by using heat energy to break down the chemical composition of the material. The carbon is not released into the air, but is stored in solid form. If the turquoise method is proven effective, it could join blue as "low carbon hydrogen", as the carbon could be permanently stored in an environmentally friendly way.
Yellow
Yellow hydrogen is another new product that is also produced by electrolysis using solar energy, similar to the process used to create green hydrogen, but with a name associated with a virtually inexhaustible source of energy - the Sun.
White or Gold
White or golden hydrogen, like other fossil fuels, occurs naturally in underground deposits of geological hydrogen. It is extracted through a process called fracking, which is widely used by oil workers around the world and is the process of drilling into the earth and sending a high-pressure mixture of water, sand and chemicals into the rock to release the gas inside. There are currently no known plans to use this type of hydrogen as an energy source.
The development of green technologies is proceeding at a rapid pace and new colours may appear in the hydrogen production palette very soon.








