New imaging method could observe body’s immune responses in real-life
Researchers have developed a new metabolic imaging technique that significantly improves the ability to study living cells. This method overcomes the common limitations of light scattering in biological tissues, allowing for deeper penetration and higher resolution imaging which could open a broad range of new opportunities for researchers developing new drugs and research into treatment efficiency without requiring to cut open body tissues.
Published on the website of the esteemed US Institute of Technology in Massachusetts (MIT), this approach uses a specialized laser to induce certain molecules in cells to emit light, offering a more natural and accurate view of tissue structure and function.
The key innovation is the use of a fiber shaper, which adapts the light by bending it to reduce scattering and enhance signal strength as it travels deeper into tissue. This development allows the laser to penetrate deeper—more than 700 micrometers, compared to the previous 200-micrometer limit—and capture clearer images. This improvement is crucial for applications such as cancer research, tissue engineering, and studying immune responses.
Unlike conventional label-based imaging, which requires tissue staining and slicing that can damage samples, this new technique is label-free, preserving the integrity of living cells while still revealing detailed molecular and structural information. The system uses a multimode fiber and a fiber shaper to precisely control light pulses and wavelengths, optimizing them for deep tissue imaging.
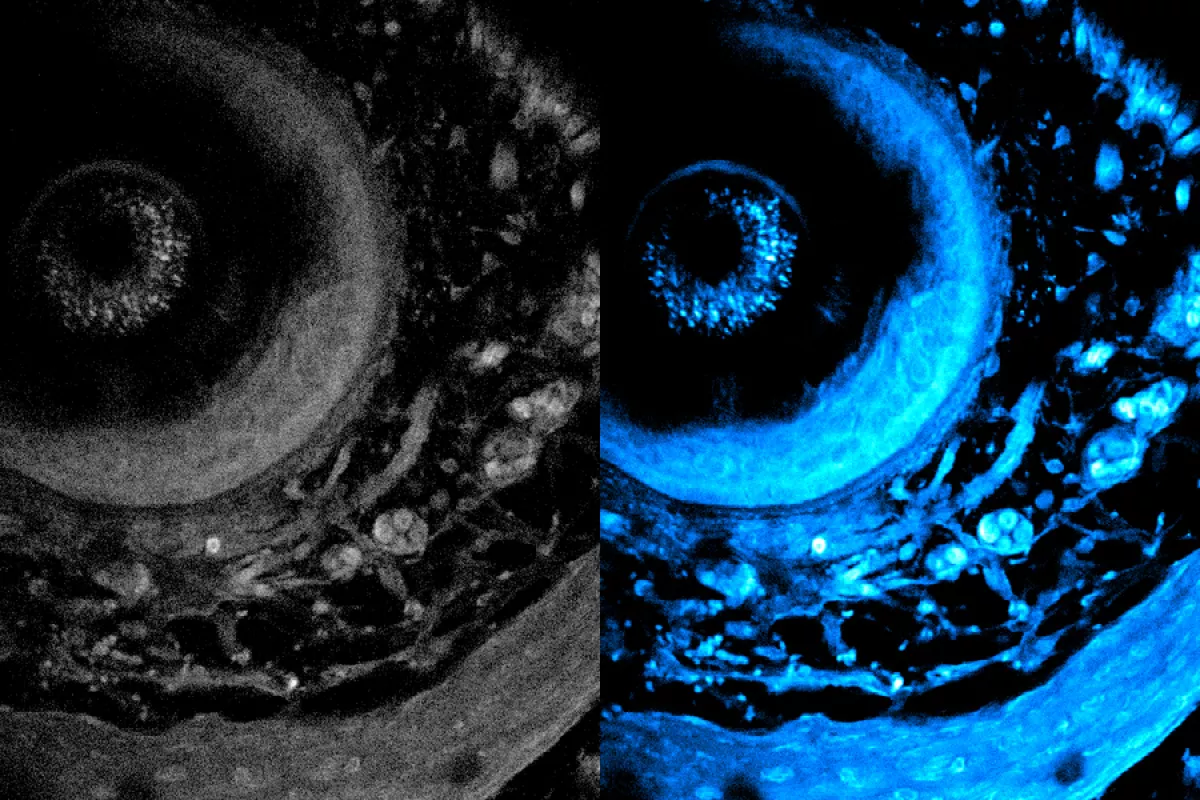
According to the institute’s update, the method has broad potential applications, including the study of organoids, which are lab-grown tissues that mimic organ functions. Researchers can now observe metabolic processes in real-time without harming the samples. The technique also promises faster imaging speeds, enabling more detailed insights into how metabolism affects cellular movement and function.
Looking ahead, the researchers plan to improve resolution further and develop algorithms that can reconstruct 3D models of biological samples. Their long-term goal is to use this technology in drug development and clinical settings, enabling real-time monitoring of drug responses and advancing our understanding of complex biological systems.
This breakthrough in metabolic imaging has garnered attention for its potential to push the boundaries of biomedical research, offering unprecedented insights into living systems and their metabolic dynamics.
By Nazrin Sadigova








